AMD and the impact on the retina
Macular degeneration or Age-related macular degeneration (AMD) is a leading cause of central vision loss in people 60 years or older. The retina is the layer that lines the back of the eye from within. The central part of the retina is the ‘Macula’, the part that is responsible for sharp vision. A healthy macula is essential for most activities including reading, writing, identifying colours, recognizing faces, and so on. The retina is a complex structure made up of ten different layers. There are two most important layers: the retinal pigment epithelium (RPE) and the photoreceptors (rods and cones). A healthy RPE is essential for the normal functioning of the retina.
With age, the RPE becomes less efficient, leading to formation of ‘Drusen’, which is a hallmark of AMD. AMD affects the macular region of the retina, leading to loss of central vision. It accounts for 8.7% of all blindness worldwide and is the most common cause of blindness in people older than 60 years. It is estimated that about 15 million to 40 million people in India suffer from AMD. AMD can have a profound impact on quality of life and independence. Affected individuals may be unable to read, write, and drive due to their loss of central vision.
There are two types of AMD-dry (Atrophic) and wet (Exudative). Dry AMD accounts for about 85% of the cases and is characterized by yellow subretinal deposits called drusen. The advanced form of the disease is called ‘Geographic atrophy’. In wet AMD, abnormal blood vessels grow under the retina that then leak blood and other fluids. Although more serious and faster to progress, wet AMD can be treated with injections of anti-VEGF into the cavity of the eye.
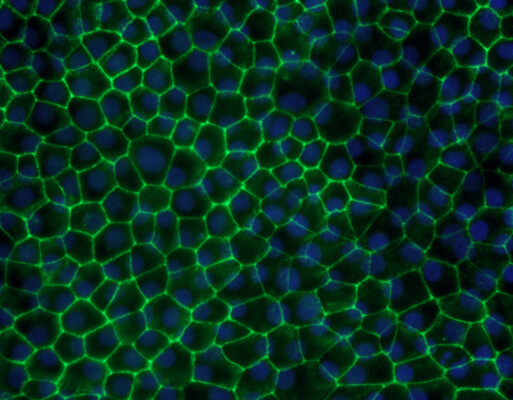
Eyecyte-RPETM
There is currently no treatment for dry age-related Macular Degeneration (AMD), the more prevalent form of AMD. Because AMD results from loss of retinal pigment epithelium (RPE), a treatment strategy currently being explored with lot of interest is to replace the lost RPE. At Eyestem, we are looking to use iPSC-derived RPE as a renewable source of cells replacing the damaged or lost RPE in an allogeneic setting. Towards this direction, we established a simple, robust, and scalable protocol to generate bonafide RPE in monolayer cultures, called Eyecyte-RPETM. The frozen Eyecyte-RPETM product showed excellent results with respect to survival, integration, and functional efficacy when transplanted into RCS rats that are born blind due to a MERTK gene mutation. Eyestem is taking this product through a CMC (Chemistry, Manufacturing and Controls) process, which involves painstaking attention to detail about the reagents used in manufacturing and associated quality controls. The product has successfully shown excellent safety and efficacy profile in animal models (primates, rats, rabbits) and is entering first in human trials.
FAQ for patients with Dry Age-related macular degeneration
The nerve tissue of the eye is called the retina. The central part of the retina is called the macula and is useful for most visual functions like reading and writing. AMD is a disease affecting the macula leading to loss of central vision.
AMD is classified into dry and wet AMD. The dry form of this disease makes up about 85% of affected patients and is characterized by yellow subretinal deposits called drusen. The advanced form of the disease is called ‘Geographic atrophy’. The dry form of AMD may also progress into the second clinical form of the disease, referred to as neovascular or wet AMD. A blood-filled membrane grows under the retina in wet AMD, leading to a rapid loss of vision.
The retina is a 10-layered structure; the retinal pigment epithelium (RPE) is one of the most important layers of the retina that acts as a foundation to support the other layers. Dry AMD is caused by a progressive damage to the RPE at the macula.
AMD is a disease affecting older people. It accounts for 8.7% of all blindness worldwide and is the most common cause of blindnessin people older than 60 years.
Affected people may notice difficulty in reading smaller print to begin with, that cannot be corrected with glasses or cataract surgery. This can progressively lead to severe difficulty to read and write, and an inability to drive. Some people may also notice distortion of their central vision, which may be a sign of wet AMD.
Advanced dry AMD can lead to legal blindness. Affected individuals may be unable to read, write, and drive. AMD can have a profound impact on quality of life and independence. However, it is important to note that most people retain good peripheral vision, which allows them to be mobile to a reasonable degree.
The progression of AMD can be assessed by a clinical examination done by a retina specialist. There are non-invasive imaging tools like fundus photos and optical coherence tomography that can assess the severity of damage to the retina.
Currently, there is no available treatment for dry AMD. However oral antioxidant/mineral supplementation has been recommended to try slowing the progression of the disease. It is also very important to stop smoking.
Wet AMD can be treated by injections of anti-VEGF agents (Ranibizumab, Bevacizumab, Aflibercept, Brolucizumab) into the cavity (vitreous) of the eye.
Stem cell treatment is not yet an approved therapy for treatment of AMD. There have been multiple reports of people losing sight due to injections of so-called stem cells into the eye. It is certainly not recommended to take stem cell treatments without exercising caution.
AMD is a multifactorial disease, caused by the interactions of multiple genes and environmental factors, with the ageing being the biggest factor. There is some genetic basis for AMD, and so family members of patients with AMD have an increased risk of developing the disease. However genetic testing for AMD is currently restricted to research purposes only.
At Eyestem Research, we have grown the retinal pigment epithelium cells that are lost in Dry AMD through a repeatable protocol. Subsequently, we have injected these cells into special animal model of degenerating retina and proven that the injected rats maintain their vision compared to non-injected animals.
We are currently working on experiments to prove that our cells are safe as per the requirements and standards accepted by the Drugs Controller General of India. Once we do that, we would be able to submit an application for starting a human trial in India. At this point, we anticipate such an application to be made in the last quarter of 2022.
Eyestem also has a product in pipeline for potential treatment of Retinitis Pigmentosa. If successful, we anticipate this product to follow the AMD product by 1-2 years. Please see our separate FAQ on Retinitis Pigmentosa for more information about this disease.
We are not looking to treat any other ophthalmic disease at this time.
The clinical trial is designed for specific end points and hence will have strict inclusion/exclusion criteria. While the clinical partners that we intend to work with will have their own network of patients, if we need more patients, a call for recruitment will be made at that time. It is important to remember that a clinical trial is not a guarantee for treatment but rather an attempt to understand the efficacy of a drug.
While we attempted to answer as many questions as we could, we are afraid we are not equipped to see patients across India. We will continue to release posts such as this for information but are not in a position to see individual patients. This is best done by a local retina specialist.
It will take anytime between 3-4 years before such a treatment is available. Each of these treatments has to go through rigorous, sequential clinical trials and regulatory approvals to prove that they indeed are efficacious and that their benefit to society justifies their approval. Unfortunately, due to the nature of scientific research, it is not possible to provide a date with absolute certainty but we at Eyestem are trying our best to get this done as fast as we can while adhering the regulatory laws of the land.
Regenerative medicine is at the cusp of a breakthrough globally and the same will happen in India as well. Our advice to patients is the following
- It is not advisable to inject unknown, unapproved cells in the eye.
- Ensure you have enough antioxidant-rich food and continue to maintain a healthy mind and body.
- Stop smoking.
- Engage with patients who have similar conditions for psychological support. Avoid Dr. Google
- Take advantage of low vision aids which are a boon in the age of electronic media.
- Follow up with your local retina specialist at intervals that you are advised.
- Continue following companies like Eyestem for news of a possible breakthrough.